- Industries
Industries
- Functions
Functions
- Insights
Insights
- Careers
Careers
- About Us
- Retail
- By Omega Team

There is a need for awareness of the greenhouse effect and the necessity to conserve the environment. There is now a green alternative in the automobile market to combat increasing fuel prices of diesel and petrol – electrically-powered cars. Electric vehicles will be a gamechanger in the transportation sector. With this comes the need to make electric vehicles more cost-effective and fuel power efficient.
An electric-vehicle battery (EVB) is a battery used to power the electric motors of a battery electric vehicle (BEV) also known as a hybrid electric vehicle (HEV). These batteries are usually rechargeable and are typically lithium-ion batteries. These batteries are specifically designed for a high ampere-hour (or kilowatt-hour) capacity, which is the battery’s unit of power generation capacity.
Electric-vehicle batteries differ from starting, lighting, and ignition (SLI) batteries as they are designed to give power over sustained periods of time and are deep-cycle batteries. Batteries for electric vehicles are characterized by their relatively high power-to-weight ratio, specific energy, and energy density; smaller, lighter batteries are desirable because they reduce the weight of the vehicle and therefore improve its performance. Compared to liquid fuels, most current battery technologies have much lower specific energy, and this often impacts the maximum all-electric range of the vehicles.
Figure 1: An Illustration of SLI
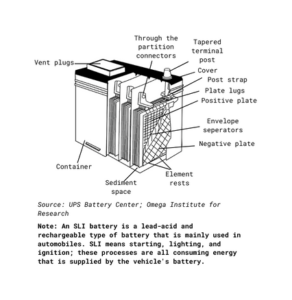
Flooded lead-acid batteries are the cheapest and, in the past, most common vehicle batteries available. There are two main types of lead-acid batteries: automobile engine starter batteries, and deep cycle batteries.
Automobile engine starter batteries are designed to use a small percentage of their capacity to provide high charge rates to start the engine. Automobile engine starter batteries are designed to use a small percentage of their capacity to provide high charge rates to start the engine, while deep cycle batteries are used to provide continuous electricity to run electric vehicles like forklifts or golf carts. Deep cycle batteries are also used as auxiliary batteries in recreational vehicles, but they require different, multi-stage charging. No lead-acid battery should be discharged below 50% of its capacity, as it shortens the battery’s life. Flooded batteries require inspection of electrolyte levels and occasional replacement of water, which gases away during the normal charging cycle.
The Battery Types
Nickel-metal Hydride
These are now considered a relatively mature technology. While less efficient (60–70%) in charging and discharging than even lead-acid, they have a specific energy of 30–80 Wh/kg, far higher than lead-acid. When used properly, nickel-metal hydride batteries can have exceptionally long lives, as has been demonstrated in their use in hybrid cars and in the surviving first-generation NiMH Toyota RAV4 EVs that still operate well after 100,000 miles (160,000 km) and over a decade of service. Downsides include poor efficiency, high self-discharge, very finicky charge cycles, and poor performance in cold weather.
Sodium Nickel Chloride
Another type is the Sodium Nickel Chloride or “Zebra” battery, it uses a molten sodium chloroaluminate (NaAlCl4) salt as the electrolyte. A relatively mature technology, the Zebra battery has a specific energy of 120 Wh/kg. Since the battery must be heated for use, cold weather does not strongly affect its operation except for increasing heating costs. They have been used in several EVs such as the Modec commercial vehicle. Zebra batteries can last for a few thousand charge cycles and are nontoxic. The downsides to the Zebra battery include poor specific power (<300 W/kg) and the requirement of having to heat the electrolyte to about 270 °C (518 °F), which wastes some energy, presents difficulties in long-term storage of charge, and is potentially a hazard.
Lithium-Ion
The most used type of battery nowadays is the Lithium-Ion battery. Lithium-ion (and the mechanically similar lithium polymer) batteries, were initially developed and commercialized for use in laptops and consumer electronics. With their high energy density and long cycle life they have become the leading battery type for use in EVs. The first commercialized lithium-ion chemistry was a lithium cobalt oxide cathode and a graphite anode first demonstrated by N. Godshall in 1979, and by John Goodenough, and Akira Yoshino shortly thereafter. The downside of traditional lithium-ion batteries includes sensitivity to temperature, low-temperature power performance, and performance degradation with age. Due to the volatility of organic electrolytes, the presence of highly oxidized metal oxides, and the thermal instability of the
anode SEI layer, traditional lithium-ion batteries pose a fire safety risk if punctured or charged improperly. These early cells did not accept or supply charge when extremely cold, and so heaters can be necessary in some climates to warm them. The maturity of this technology is moderate. The Tesla Roadster (2008) and other cars produced by the company used a modified form of traditional lithium-ion “laptop battery” cells.
Recent EVs are using new variations on lithium-ion chemistry that sacrifice specific energy and specific power to provide fire resistance, environmental friendliness, rapid charging (as quickly as a few minutes), and longer lifespans. These variants (phosphates, titanates, spinels) have been shown to have a much longer lifetime, with A123 types using lithium iron phosphate lasting at least more than 10 years and more than 7000 charges/discharge cycles, and
LG Chem expecting their lithium-manganese spinel batteries to last up to 40 years.
Much work is being done on lithium-ion batteries in the lab. Lithium vanadium oxide has already made its way into the Subaru prototype G4e, doubling energy density. Silicon
nanowires, silicon nanoparticles, and tin nanoparticles promise several times the energy density in the anode, while composite and superlattice cathodes also promise significant density improvements.New data has shown that exposure to heat and the use of fast charging promote the degradation of Lithium-ion batteries more than age and actual use, and that the average electric vehicle battery will retain 90% of its initial capacity after 6 years and 6 months of service. For example, the battery in a Nissan LEAF will degrade twice as fast as the battery in a Tesla, because the LEAF does not have an active cooling system for its battery.
Figure 2: 2019 BNEF Battery PriceSurvey
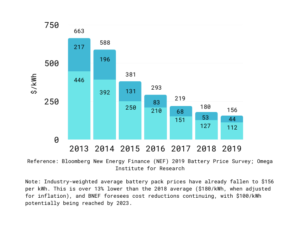
The above graph shows the cost of batteries over the years past. It can be seen that over the years, the prices have fallen continuously for all battery types, except the Li-ion batteries which had a spike in 2013, followed by a downfall. This can be due to the rise and demand and the price inelastic nature.
With being the latest to get introduced in the market, Li-ion seems to be best in terms of efficiency too. Li-ion has better miles to life ratio than the lead-acid battery. In 2016, the Li-Ion battery is estimated to last over 10 years of battery life, while being able to run for over 1,000,000 miles, outperforming all other battery types.
The Future of EVB
While the Li-ion battery will continue to progress, it will be solid-state and lithium-silicon technologies that will be the real EVB game changer. Ongoing and significant improvements in battery technology will pave the way for an installed EV base of 100 million by 2028, according to the global tech market advisory firm, ABI Research. Between 2023 and 2025, continually increasing silicon in batteries to the point where developments will enable silicon-dominant anodes.
Given the research taking place in lithium-silicon batteries and the increasing percentage of silicon in EV batteries, ABI Research believes this is the next logical step. Silicon-dominant batteries would likely enable energy densities of up to 400 Wh/kg by 2025. Most vehicles using this technology will likely have to charge powers of 300 kilowatts.
Silicon dominant anodes will be the primary solution until 2026 – at the earliest – when solid-state battery architectures will start to be deployed and reach commercialization. Solid-state batteries will enable energy densities of at least 500 Wh/kg, offer 500 kW+ charging power.
Subscribe
Select topics and stay current with our latest insights
- Functions